According to the EUROPE DIRECT CONTACT CENTER consultation response summary (full response attached):
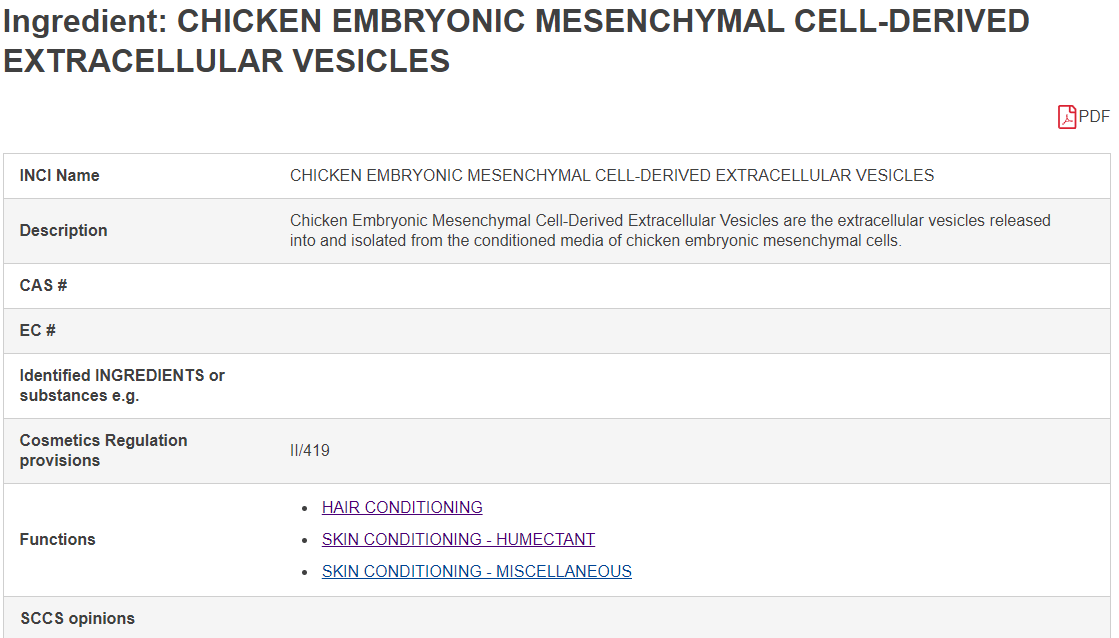
All cosmetics related to animal sources and derived ingredients will be labeled ANNEX II/419. However, this is only for reference and not as a basis for restriction/prohibition.
Furthermore, according to cosmetics regulations (1223/2009), the responsible person must ensure the safety of cosmetics on the market and assume corresponding responsibilities when using or not using such ingredients, and demonstrate whether the entries indicated in CosIng are related to these ingredients.
The ingredient “chicken embryonic mesenchymal cell-derived extracellular vesicles” from ExoGiov comes from SPF (Specific Pathogen Free) chicken eggs and does not fall under the categories mentioned in ANNEX II/419. It also has a safety report from a third-party inspection company, ensuring its safety for use in cosmetics.
Europe Direct Contact Centre <EuropeDirectContactCentre@edcc.ec.europa.eu>
Your Europe Direct reply no #3662921
Dear Mr S. H. Lee,
Thank you for contacting the Europe Direct Contact Centre.
We have consulted the Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs (DG GROW). Please find below the answer to your question.
“CosIng has only informative value, it is not a legally binding instrument. In addition, entry II/419 refers to ‘Category 1 material and Category 2 material as defined in Articles 4 and 5 respectively of Regulation (EC) No 1774/2002 of the European Parliament and of the Council ( 3 ), and ingredients derived therefrom”. This is added for information purposes. And it does not service as a restriction/prohibition as such.
According to the Cosmetics Regulation (1223/2009), the responsible person must ensure the safety of the cosmetic product placed on the market and has the respective responsibility in using or not such ingredients in cosmetics and attest whether the entry indicated in CosIng is relevant to these ingredients or not.
We hope you find this information useful. Please contact us again if you have other questions about the European Union, its activities or institutions.”
We hope you find this information useful. Please contact us again if you have other questions about the European Union, its activities or its institutions.
With kind regards,
Europe Direct Contact Centre
We answer all questions about the EU




